Autoimmune hepatitis isn’t something you can catch from someone else. It’s not caused by alcohol, viruses, or poor diet. It happens when your own immune system turns on your liver, attacking it like it’s an invader. This chronic inflammation can slowly destroy liver tissue, leading to scarring, cirrhosis, or even liver failure - if left untreated. The good news? We know how to stop it. And the two most important tools in that fight are steroids and azathioprine.
How Do Doctors Diagnose Autoimmune Hepatitis?
There’s no single test for autoimmune hepatitis. Instead, doctors piece together clues from blood work, imaging, and a liver biopsy. It’s like solving a puzzle where every piece matters. Blood tests usually show high levels of liver enzymes - ALT and AST - often five to ten times above normal. That’s a red flag. But many conditions cause that. So next, they look for autoantibodies. Antinuclear antibodies (ANA) or smooth muscle antibodies (SMA) are found in about 80% of cases. Another type, LKM1, shows up in the other 20%. But here’s the key change: as of the 2025 European Association for the Study of the Liver guidelines, these antibody types no longer determine treatment. They’re just diagnostic markers, not treatment guides. IgG levels, a type of immune protein, are also checked. If they’re more than 1.5 times the upper limit of normal, that adds weight to the diagnosis. But the real confirmation comes from a liver biopsy. A small sample of liver tissue is taken with a thin needle, guided by ultrasound. Under the microscope, pathologists look for something called interface hepatitis - inflammation right where the liver’s portal areas meet the healthy tissue. This pattern is unique to autoimmune hepatitis. The biopsy must show this in at least 20 portal tracts to meet diagnostic standards. Doctors use a scoring system called the Revised International Autoimmune Hepatitis Group (IAIHG) scale. Points are added for symptoms, blood markers, biopsy findings, and ruling out other causes like hepatitis B or C. A score above 15 means probable AIH. Above 20? Definite AIH.Why Steroids Are the First Line of Defense
Prednisone (or its active form, prednisolone) has been the backbone of AIH treatment since the 1970s. It works fast. In 80-90% of patients, liver enzyme levels drop noticeably within two weeks. That’s how you know the immune system is being suppressed. The starting dose is usually 0.5 to 1 mg per kilogram of body weight - capped at 60 mg per day. For most adults, that’s about 30-40 mg daily at first. The goal isn’t to stay on this high dose forever. It’s to get the inflammation under control, then taper down. By week 6 to 8, doctors aim to reduce the dose to 10-15 mg per day. That’s still enough to keep the immune system quiet but low enough to start reducing side effects. About 70% of patients on steroid-only treatment develop problems like weight gain, mood swings, high blood sugar, or bone thinning. That’s why steroids are almost never used alone long-term.The Role of Azathioprine: The Steroid-Sparing Partner
Azathioprine (brand name Imuran, or generic azathioprine) is the other half of the equation. It’s an immunosuppressant that doesn’t work fast - it takes weeks to months to kick in. But once it does, it lets doctors cut the steroid dose by 70-80% within six months. The typical starting dose is 50 mg per day, then slowly increased to 1-2 mg per kg of body weight - usually capped at 150 mg daily. It’s taken once a day, often with food to reduce stomach upset. The real win? Combination therapy cuts steroid side effects in half. Instead of 70% of patients suffering complications, it drops to 30%. That’s huge when you’re talking about long-term treatment. But azathioprine isn’t risk-free. About 35% of patients get nausea or diarrhea. Around 12% develop bone marrow suppression - meaning lower white blood cell counts, which raises infection risk. And in 0.3% of people, there’s a dangerous, life-threatening drop in blood cells because they lack an enzyme called TPMT. That’s why testing for TPMT before starting azathioprine is now standard. It’s a simple blood or genetic test that costs $250-$400 in the U.S. It’s done in 89% of academic centers now, though only 45% of community clinics still skip it. Skipping this test is like driving blindfolded.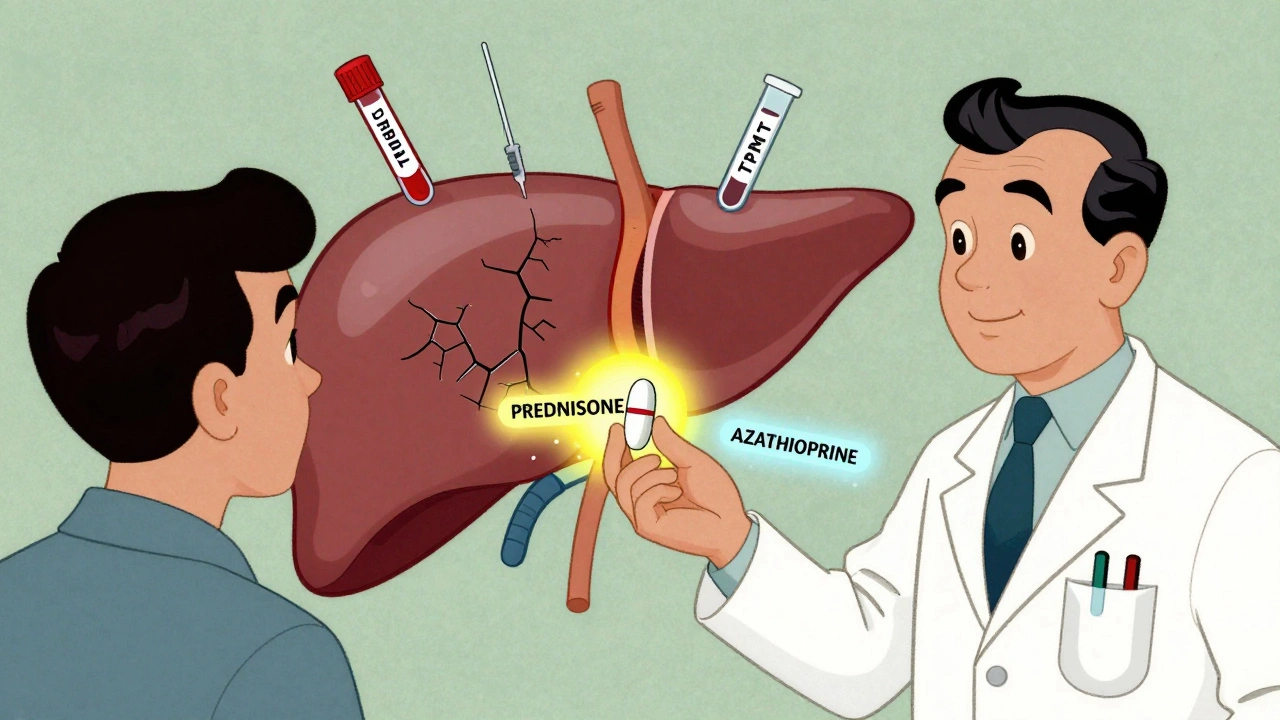
How Long Do You Stay on Treatment?
This is where things get personal. Most people stay on treatment for years - sometimes for life. Complete biochemical response - meaning ALT, AST, and IgG levels return to normal - happens in 60-80% of patients within 18 to 24 months. But that doesn’t mean the disease is gone. It means the immune system is being kept in check. A repeat liver biopsy after 2-3 years shows histological remission - meaning the liver tissue has healed - in 50-70% of patients. That’s a powerful sign. Some patients even see fibrosis reverse, moving from stage F3 (advanced scarring) back to F0 (no scarring). But here’s the catch: if you stop treatment, relapse is likely. Between 50% and 90% of patients relapse within months of quitting. That’s why doctors rarely suggest stopping unless you’ve been in deep remission for at least two years, and even then, it’s done slowly over 6-12 months. Only about 45% of people who try to stop treatment stay off it without relapse. And 70% of those relapses happen within three months of stopping.What If the First Treatment Doesn’t Work?
About 10-15% of patients don’t respond well to steroids and azathioprine. That’s called treatment failure. Signs? Liver enzymes stay above twice the normal level after 12-18 months. When that happens, doctors switch to second-line drugs. Mycophenolate mofetil (CellCept) is the most common alternative. It’s taken twice daily, usually 1-1.5 grams per dose. It’s effective in about 60% of those who fail first-line therapy. Other options include calcineurin inhibitors like tacrolimus or cyclosporine. These are powerful and require careful blood monitoring. New drugs are on the horizon. Obeticholic acid (Ocaliva), approved for primary biliary cholangitis, is now being tested in AIH. Early results show a 42% complete response rate - better than the 28% seen with standard therapy. JAK inhibitors like tofacitinib and monoclonal antibodies targeting interleukin-6 are also showing promise in early trials. These could be game-changers for people who can’t tolerate current treatments.What You Need to Do Before and During Treatment
Before starting any immunosuppressant, you must be tested for hepatitis B. About 15-20% of people carry the virus silently. If you’re on steroids or azathioprine, that virus can wake up and cause severe liver damage. If you test positive, you’ll need antiviral meds like tenofovir before starting AIH treatment. You should also get vaccinated for hepatitis A and B - before treatment starts. Once you’re immunosuppressed, vaccines don’t work as well. In healthy people, the hepatitis B vaccine is 90% effective. In people on steroids, it’s only 40-60%. Monitoring is non-negotiable. Blood tests for liver enzymes should be done every 2-4 weeks in the first few months. Once stable, every 3 months. IgG levels are checked quarterly. Blood counts are checked monthly for the first six months on azathioprine.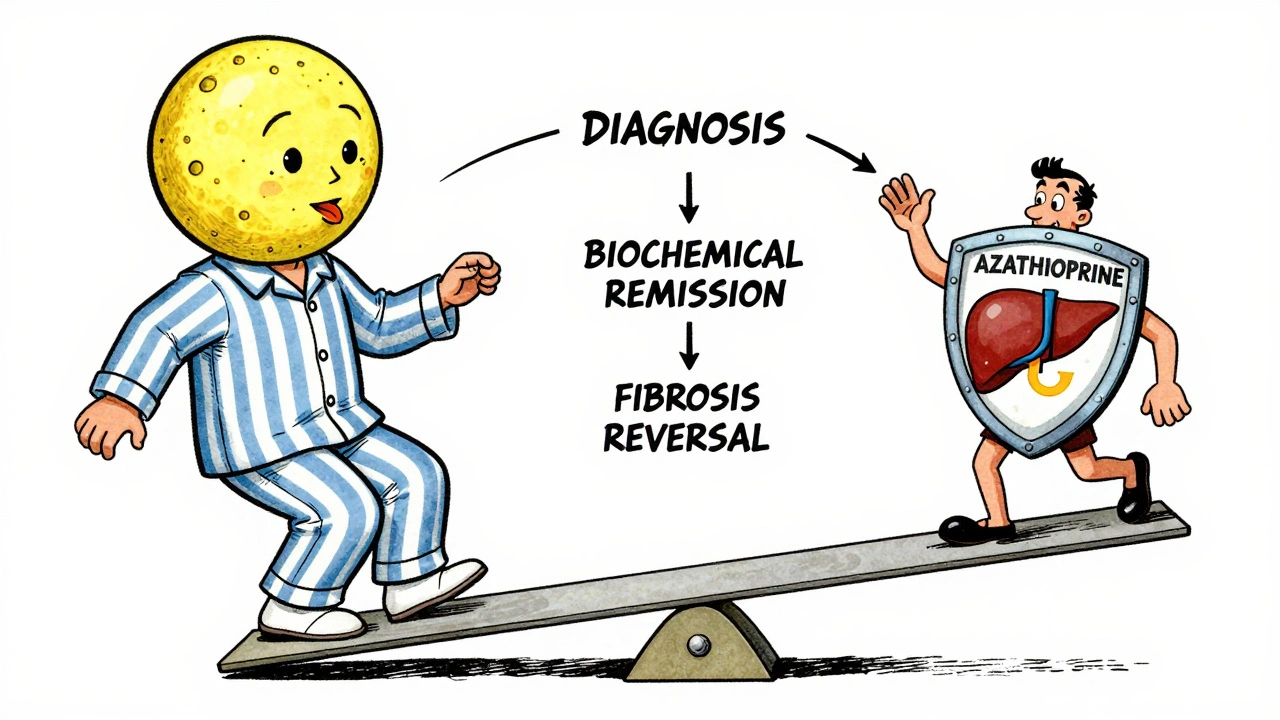





Comments
This is one of the clearest explanations of AIH I’ve ever read. The way you broke down the biopsy criteria and the steroid-azathioprine combo? Perfect. I’ve been managing this for 8 years, and even I learned something new about the 2025 EASL updates. Thank you for writing this.
The clinical precision here is commendable. The emphasis on TPMT testing before azathioprine initiation reflects a necessary evolution in patient safety protocols. It is encouraging to see guidelines adapt to evidence rather than tradition.
Okay but what if the real reason AIH is rising is because of the 5G towers in every town? I read a study (not peer-reviewed, but on a forum) that said EMF radiation triggers autoimmune misfires. And don’t even get me started on the glyphosate in our water. My cousin’s liver went south after she started drinking kombucha. Coincidence? I think not. 🤯
Hi. I’m a nurse who’s seen this play out in real life. I want to say: if you’re scared of the side effects, you’re not alone. But please, don’t quit. I had a patient - 52, mom of two - went from ALT 1200 to normal in 10 weeks. She cried when she saw her biopsy results. It’s not magic. It’s medicine. And it works.
OMG YES THIS!! 🙌 I was on 40mg prednisone and felt like a balloon with legs 😭 But once I switched to azathioprine? Life changed. I started hiking again. I slept through the night. I even got my hair back!! (well, most of it 😅) Don’t give up - your liver is fighting for you too 💪❤️
You say steroids are the backbone but you never mention how many people end up with osteoporosis or diabetes. You sound like a pharma rep. The real solution is fasting and turmeric. Everyone knows that. You're just selling fear to keep people on pills.
These guidelines are a joke. In Canada, we don’t waste money on TPMT tests. We just watch the blood counts. If the patient can’t handle it, they’re not strong enough. Medicine isn’t for the weak. Steroids work. That’s it.
Thank you for highlighting the importance of longitudinal monitoring. The shift from 6-month to 12-month response evaluation aligns with real-world clinical variability. Many patients exhibit delayed biochemical improvement, and premature treatment alteration risks unnecessary escalation. This is evidence-based medicine at its best.
Man, I wish I’d had this when I was first diagnosed. I thought I was dying. I stopped taking my meds for two months because I hated the moon face. My ALT shot up to 1500. My doctor looked at me like I’d betrayed him. But then I got back on track - slow taper, azathioprine, and a whole lot of yoga. Now I’m off steroids, just 50mg azathioprine, and I’m running marathons. It’s not easy. But it’s worth it. You got this.
Why are we letting Big Pharma dictate our treatment? In America, we’re forced into this steroid-azathioprine combo because it’s profitable. In Europe, they use herbal remedies and diet protocols. We’re being turned into lab rats. Wake up.
My sister had AIH. She died of liver failure at 34. They told her to take steroids. She refused because of the weight gain. Now I’m terrified to start mine. Why isn’t there a better option? Why do we have to suffer like this?
Actually, the 2025 guidelines still use autoantibodies to stratify prognosis - you just don’t change the drug based on them. You said they’re not treatment guides, but they’re still prognostic markers. You’re oversimplifying. Also, Obeticholic acid isn’t approved for AIH yet. Don’t mislead people.
There’s something beautiful about how the liver can heal itself if you just give it a chance. I used to think my body was broken. Now I see it as a warrior. I’m not cured - but I’m alive. And I’m not just surviving. I’m living. That’s the real win. Keep going. You’re not alone.
Why is everyone so obsessed with azathioprine?? I had a bad reaction and switched to mycophenolate - and guess what? My white blood cell count went from 2.1 to 5.8 in 3 weeks! Why don’t docs just tell you this upfront? Also, my doctor didn’t test my TPMT - I had to ask for it myself. Stupid.