Short answer many people won’t expect: enclomiphene is built to nudge the ovaries to ovulate. After menopause, the ovaries no longer respond. So for typical post-menopausal symptoms-hot flushes, night sweats, brain fog, vaginal dryness-this drug doesn’t solve the core problem. If you clicked hoping for a discreet workaround to standard hormone therapy, I’ll save you time: the evidence doesn’t support enclomiphene for post-menopausal women, and it isn’t approved for that use in the US, EU, or Australia in 2025.
- TL;DR: There’s no quality evidence that enclomiphene improves post-menopausal symptoms or bone health; it isn’t approved for women after menopause.
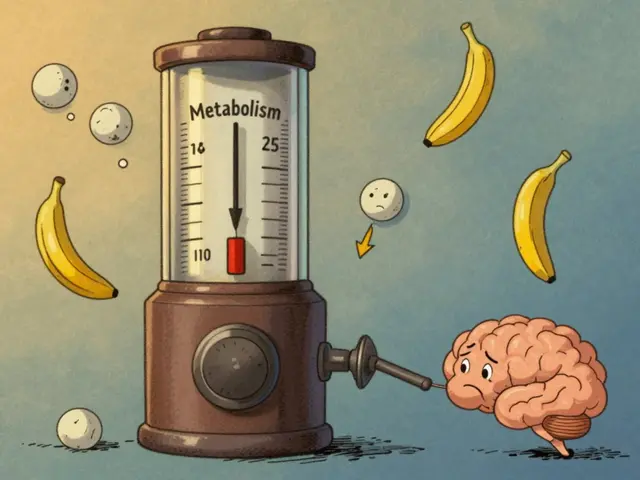
- Mechanism mismatch: It tries to make the pituitary shout louder at ovaries. After menopause, the ovaries won’t answer.
- Risks exist (e.g., hot flushes, mood changes, visual symptoms, possible clot risk typical of SERMs) without proven benefits in this group.
- Better options: Menopausal hormone therapy (MHT) is most effective for vasomotor symptoms; non-hormonal options like fezolinetant and certain SSRIs/SNRIs also work.
- Red flags: Clinics selling compounded enclomiphene to “fix menopause” are running off-label experiments. Ask for evidence; you won’t see strong data.
What enclomiphene is, how it works, and why it doesn’t fit post‑menopause
Enclomiphene is one of the two isomers in clomiphene citrate (the other is zuclomiphene). Clomiphene has been used for decades to induce ovulation in premenopausal women with irregular cycles. Enclomiphene selectively blocks estrogen’s negative feedback in the hypothalamus, pushing the pituitary to release more LH and FSH. In a cycling woman, that nudge can trigger follicle development and ovulation.
After menopause, the biology is different. The ovaries are no longer producing follicles that respond to LH/FSH. Pituitary hormones are already high (your lab results often show elevated FSH and LH). Turning up that signal even further doesn’t restart ovarian function. That’s the basic mismatch: enclomiphene tries to influence a pathway that has already reached its limit post-menopause.
What symptoms are we trying to fix here? The usual culprits are vasomotor symptoms (hot flushes, night sweats), sleep disturbance, mood changes, genitourinary issues (vaginal dryness, painful sex, urinary urgency), and long-term bone loss. These come from estrogen deficiency and neuroendocrine changes in the brain. Enclomiphene does not replace estrogen, and it doesn’t target the neurokinin pathways that drive hot flushes. So it’s not built for the job.
Compare that to menopausal hormone therapy (MHT), which directly supplies estrogen (transdermal patches, gels, or oral forms), often with a progestogen if you have a uterus. That addresses the root cause and is still the most effective treatment for hot flushes and night sweats per major guidelines (e.g., The North American Menopause Society 2022 position statement). For women who can’t or don’t want hormones, a newer class-neurokinin 3 receptor antagonists like fezolinetant-reduces flushes by targeting the hypothalamic KNDy neuron pathway. SSRIs/SNRIs such as venlafaxine and escitalopram can also help.
Could enclomiphene help in any post-menopausal scenario? Very unlikely. In theory, a SERM might offer tissue-selective benefits, but enclomiphene is not the SERM we use for bones or breast risk reduction. Raloxifene is the SERM with bone data and breast cancer risk reduction in post-menopausal women. Tamoxifen is used for breast cancer treatment and prevention in selected women. Enclomiphene doesn’t have clinical outcome data in post-menopausal women showing improvements in vasomotor symptoms, bone density, or vaginal health.
One more practical angle: side effects seen with clomiphene-like drugs-hot flushes, visual blurring, mood lability-can actually overlap with or worsen menopausal complaints. That’s not what you want when you’re trying to sleep through the night again.

Evidence, safety, and regulation in 2025
Let’s cover what’s known and what’s noise.
Evidence in post-menopausal women: There are no robust randomized trials showing enclomiphene benefits for post-menopausal symptoms or long-term outcomes. You’ll find studies in men with hypogonadism (where it attempts to raise testosterone by stimulating LH/FSH to the testes), and classic clomiphene studies in premenopausal women for ovulation induction. That’s a different population and purpose. If a clinic is leaning on male hypogonadism data to sell enclomiphene to post-menopausal women, that’s a shaky extrapolation.
Regulatory status: As of 2025, enclomiphene is not FDA-approved in the United States for any indication, and it isn’t approved by the European Medicines Agency or Australia’s TGA for use in post-menopausal women. In the mid-2010s, the FDA issued Complete Response Letters for an enclomiphene product aimed at male hypogonadism; approval didn’t happen. Some telehealth clinics compound enclomiphene for men off label, but that doesn’t translate to a green light for women after menopause, and compounded drugs don’t go through the same approval process.
Safety profile: While post-menopausal data are thin, we can infer potential risks from clomiphene and SERM class effects:
- Vasomotor symptoms: Hot flushes are common with SERMs like clomiphene and tamoxifen. That’s the opposite of what you want if flushes are your main issue.
- Visual effects: Clomiphene can cause visual disturbances (blurred vision, scotomas). These are uncommon but important because they can be persistent in rare cases.
- Mood and sleep: Some users report mood swings, irritability, and insomnia. Hard to separate from menopausal symptoms-but they can stack.
- VTE risk: SERMs can increase risk of venous thromboembolism (blood clots). Raloxifene and tamoxifen carry this class warning. Enclomiphene’s specific risk in post-menopausal women isn’t quantified, but caution is warranted, especially in women with personal or family history of VTE.
- Endometrium: Unclear for enclomiphene in this age group, but SERMs vary; tamoxifen can stimulate endometrium, raloxifene doesn’t. Without strong data, we can’t assume a safe profile for uterine tissue.
- Lipids and liver: Clomiphene can shift lipid profiles and rarely affect liver enzymes. You’d want baseline labs if anyone ever prescribed a SERM off label in this population.
How does this compare with established options? The North American Menopause Society (2022), international menopause societies, and most national bodies say: MHT is first-line for moderate-to-severe vasomotor symptoms in healthy women under 60 or within 10 years of menopause, absent contraindications. Transdermal estradiol at the lowest effective dose, with a progestogen if you have a uterus, is common. For those who can’t or prefer not to use hormones, fezolinetant has solid RCTs reducing hot flush frequency and severity, and SSRIs/SNRIs have consistent (though smaller) effects. Local vaginal estrogen (or DHEA/prasterone) works very well for genitourinary symptoms and has minimal systemic absorption.
| Therapy | Main purpose | Hot flush relief | Bone benefit | Genitourinary relief | Key risks | 2025 approval snapshot (US/EU/AUS) |
|---|---|---|---|---|---|---|
| Enclomiphene | Stimulate gonadotropins; studied in male hypogonadism | No solid evidence post-menopause | No outcomes data | No | Possible hot flushes, visual symptoms, SERM-class VTE risk | Not approved for women; not approved broadly |
| Clomiphene citrate | Ovulation induction (premenopausal) | No | No | No | Hot flushes, visual effects, mood changes | Approved for ovulation induction; not for menopause |
| Transdermal/oral estradiol ± progestogen (MHT) | Replace estrogen; relieve VMS | Yes, strongest effect | Helps prevent early post-menopause bone loss | Local vaginal estrogen best; systemic helps some | Breast/cholecystitis/stroke risks vary by route, dose, age | Approved; first-line for eligible women |
| Fezolinetant (NK3R antagonist) | Targets hypothalamic KNDy neurons | Yes, proven in RCTs | No | No | Liver enzyme monitoring, headache, abdominal pain | FDA approved 2023; regulatory status varies by country |
| SSRIs/SNRIs (e.g., venlafaxine, escitalopram) | Non-hormonal VMS relief | Yes, moderate effect | No | No | Nausea, sleep changes, sexual side effects | Approved (psychiatric indications); used off label for VMS |
| Raloxifene (SERM) | Bone density, breast risk reduction | No (can worsen) | Yes | No | VTE risk, leg cramps | Approved for osteoporosis prevention/treatment |
| Local vaginal estrogen or DHEA | Genitourinary syndrome of menopause | No (systemic symptoms minimal) | Local effects only | Yes, strong effect | Minimal systemic risks | Approved; widely recommended |
Bottom line on safety and regulation: when a therapy lacks approval, lacks outcome data, and has plausible class risks, using it outside a trial-especially when good options exist-just isn’t a smart bet.

What to do instead: decision guide, better options, and FAQs
Here’s a practical way to move forward if you were eyeing enclomiphene as a workaround.
Step-by-step decision guide:
- Pin down your top symptoms. Are hot flushes the worst? Is sleep the main issue? Is painful sex or urinary urgency your daily problem? Your treatment should match your top symptom.
- Check timing and risk factors. Under 60 or within 10 years since your final period, with no contraindications? You’re likely a good candidate for MHT per NAMS 2022 and other guidelines. Past breast cancer, recent clot, stroke, or uncontrolled blood pressure? You may need non-hormonal routes.
- Pick the right tool:
- Vasomotor symptoms: MHT (transdermal estradiol preferred for many), or non-hormonal options (fezolinetant, venlafaxine, escitalopram, gabapentin) if hormones aren’t for you.
- Genitourinary symptoms: Local vaginal estrogen or DHEA is highly effective and low risk.
- Bone health: Weight-bearing exercise, adequate calcium and vitamin D, plus medications like bisphosphonates or raloxifene if you meet criteria.
- Plan monitoring. Whatever you choose, set up a follow-up at 6-12 weeks to check symptom response and side effects. For fezolinetant, you’ll need liver enzyme checks. For MHT, track blood pressure, breast screening as per age group, and symptom relief.
- Avoid off-label detours that add risk without benefit. Enclomiphene for post-menopause is exactly that.
Simple rules of thumb:
- If your ovaries can’t respond, a drug that shouts at them won’t fix your symptoms.
- When there’s no approval and no trials in your group, assume risk without benefit until data say otherwise.
- Match treatment to the symptom: systemic for hot flushes; local for vaginal symptoms; bone meds for osteoporosis risk.
Red flags to watch in clinics or ads:
- “Enclomiphene balances female hormones after menopause.” That’s not how it works.
- Claims based on male hypogonadism or premenopausal fertility studies applied to post-menopausal women.
- No reference to major guidelines (NAMS, Endocrine Society, RANZCOG) or regulatory approvals.
- Compounded-only offerings with subscription models, no lab monitoring, and no plan for side effects.
Cost sanity check:
- MHT: Generic estradiol patches and micronized progesterone are often affordable; cost varies by brand and pharmacy.
- Fezolinetant: Newer and pricier; worth it if you need non-hormonal relief and it works for you.
- SSRIs/SNRIs: Inexpensive generics; dose adjustments are common to balance relief and side effects.
- Local vaginal estrogen: Low-dose products are often used 2-3 times per week after loading; long-term costs are moderate and benefits strong.
Mini‑FAQ
Q: Is enclomiphene ever used in post-menopausal women for bone health?
A: No quality outcome data support that. If bone protection is your goal, proven options include bisphosphonates, denosumab, or raloxifene (if appropriate), plus lifestyle and calcium/vitamin D.
Q: Could enclomiphene help with low libido after menopause?
A: Libido is complex-hormones, mood, sleep, relationship, and pain all matter. Enclomiphene doesn’t treat genitourinary discomfort or raise estrogen. Consider local vaginal estrogen or DHEA for pain/dryness, address sleep and mood, and discuss testosterone therapy only if you meet criteria for hypoactive sexual desire disorder in a specialist setting.
Q: What about using clomiphene instead?
A: Same logic. It’s an ovulation-induction drug. Not appropriate for typical post-menopausal symptoms and can worsen hot flushes.
Q: I can’t take estrogen. Do I have any good options?
A: Yes. Fezolinetant (where available), venlafaxine, desvenlafaxine, escitalopram, or gabapentin can help hot flushes. For vaginal symptoms, local therapies work very well and are low risk. Pelvic floor physio and moisturisers/lubricants help too.
Q: Is transdermal estrogen safer than oral?
A: For many women, transdermal routes are linked to a lower risk of venous clots and stroke compared with some oral regimens, especially at lower doses. Your personal risk profile matters, so weigh this with your clinician.
Q: I’m more than 10 years past menopause. Is MHT off the table?
A: Not always, but the balance of benefits and risks shifts. Non-hormonal options often take the lead. If MHT is considered, it’s usually at low dose with careful monitoring and a clear indication.
Quick checklist to take to your appointment:
- My top two symptoms: __________ and __________
- Years since last period: __________
- Medical history: breast cancer, clots, stroke, migraine with aura, liver disease, uncontrolled hypertension (circle any)
- Current meds/supplements: __________
- Preferences: hormonal vs non-hormonal, patch vs pill vs gel
- What I’m worried about: weight, sleep, brain fog, sex, bone, heart
- Goal for the next 8 weeks: e.g., reduce night sweats by 70%, sleep 6-7 hours, pain-free sex
Pitfalls to avoid:
- Chasing unapproved, low-evidence options (like enclomiphene) when proven, safer therapies exist.
- Skipping the progestogen if you have a uterus and use systemic estrogen-protecting the endometrium matters.
- Stopping too soon: give treatments a fair trial window (e.g., 4-8 weeks for many VMS therapies) unless side effects are significant.
- Ignoring lifestyle pillars: exercise, sleep hygiene, alcohol moderation, and hot-flush triggers (spicy food, hot rooms) still help.
When to seek urgent care:
- Sudden chest pain, shortness of breath, swelling/pain in a leg (possible clot).
- New severe headache with neurological changes.
- Visual disturbances that don’t resolve quickly.
Notes on guidelines and credibility: The North American Menopause Society’s 2022 position statement recognises MHT as the most effective treatment for vasomotor symptoms. The Endocrine Society’s clinical practice guidance similarly supports individualised hormone therapy with attention to timing and risk. For non-hormonal therapy, fezolinetant earned FDA approval in 2023 on the strength of randomised trials. None of these bodies recommend enclomiphene for post-menopausal women. If your clinician suggests it, ask them to show an RCT in post-menopausal women with symptom outcomes; you won’t find one.
Next steps if you’re considering treatment now:
- Book a focused visit. Bring the checklist above. Ask directly about MHT eligibility and non-hormonal options.
- Decide on one primary therapy to trial. Set a measurable goal and timeframe.
- Plan follow-up and monitoring (liver tests for fezolinetant; blood pressure and breast screening schedules for MHT; symptom diary for any option).
- Reassess at 6-12 weeks. If it’s not working, adjust dose, switch class, or combine with local therapy for vaginal symptoms.
Last word on enclomiphene in this context: if a drug isn’t designed for your biology after menopause, lacks trials in your group, and isn’t approved, it’s not the shortcut. The good news is you do have effective, safer tools-with real data behind them-to get your life back.





Comments
OMG I just spent $800 on this enclomiphene stuff from some Telegram clinic 😭 they told me it's 'natural estrogen rebalancing'... now I'm having hot flashes worse than before and my vision is blurry. why do people sell snake oil like this???
so wait… enclomiphene is like a shout at a deaf person? lol that’s a good way to put it. i thought it was some magic hormone pill. my aunt took it and said she felt ‘wired’ but no better. guess the ovaries just checked out.
Thank you for this exceptionally clear, evidence-based breakdown. As a physician, I see too many patients misled by aggressive marketing of unapproved compounds. The comparison table alone should be mandatory reading for every menopausal woman considering off-label options. The risks are real, the alternatives are validated, and the ethical obligation to guide patients away from unproven therapies cannot be overstated.
Let me guess - Big Pharma doesn't want you to know this because they can't patent a hormone your body already makes. The FDA's 'Complete Response Letter'? That's just a delay tactic. Compounded enclomiphene is cheaper, works better, and they're silencing it. They don't want women to have control over their own hormones - they want you hooked on their expensive patches and pills. Look at the history of estrogen suppression in the 90s - same playbook.
This post feels like a lifeline. I spent six months chasing ‘biohacks’ and ‘hormone balancing’ before finding a menopause specialist who actually listened. I was desperate. I tried everything from maca powder to IV drips. The truth is, menopause isn’t a problem to be fixed - it’s a transition to be supported. And the support we need isn’t in a pill from a shady website. It’s in the right doctor, the right tools, and the patience to find them.
Bro… what if the ovaries aren’t the problem? What if it’s the hypothalamus that’s been gaslit by estrogen for 40 years? Enclomiphene is just the body’s way of screaming back. They don’t want you to know that menopause isn’t natural - it’s a corporate-designed shutdown. The real cure? Cold exposure, red light, and ancestral fasting. But hey, go ahead and take your patch. I’ll be over here optimizing my pineal gland.
So you're telling me American women are being scammed by Indian clinics selling lab-made pills to fix what’s natural? That’s not medicine - that’s cultural imperialism. We’ve got real medicine here. Stop letting foreign quacks profit off our pain.
As a medical researcher in Lagos, I’ve seen this pattern across Africa too - menopause is treated like a disease needing a pill, not a life stage needing support. Enclomiphene? It’s a Band-Aid on a broken leg. We need community education, not online clinics. Local herbalists have better data on black cohosh and moringa than these compounding pharmacies. Let’s build real systems - not profit-driven scams.
ok but what if i just want to feel hot again? like, emotionally hot? not just physically. this whole post is so dry. i miss my period. i miss my boobs. i miss being able to cry without it being called ‘hormonal’. why is no one talking about the grief? just give me the magic pill already.
You think this is bad? Wait until you’re 70 and your spine looks like a crumpled paper bag. Enclomiphene might not help hot flashes, but raloxifene? That’s the real hero. You think the pharmaceutical industry is evil? They didn’t invent osteoporosis - menopause did. And if you’re not taking something to protect your bones, you’re not being responsible. Stop chasing feel-good nonsense and look at the data.
Just wanted to add - if you're on SSRIs for hot flashes, don't stop them cold turkey. I did and had electric shock dreams for a week. Taper slow. Also, gabapentin works better than people admit. I took 300mg at night and my night sweats vanished. No magic, just science.
They say the ovaries are done. But what if they’re just tired? Like a car that’s been revved too hard for too long. Maybe we’re not supposed to restart the engine - maybe we’re supposed to learn how to ride a bicycle instead. Enclomiphene is the gas pedal. Fezolinetant? That’s the handlebars. And MHT? That’s the bike you’re too scared to ride because someone told you it’ll crash. But maybe… maybe it’s the only way to feel free again.
While I appreciate the clinical precision of this analysis, I find it deeply disheartening that the medical establishment continues to frame menopause as a pathological condition requiring pharmacological intervention. The very language of ‘symptoms’ and ‘treatments’ reinforces the notion that a woman’s body post-45 is inherently broken. Might we not consider, instead, a philosophical reorientation - one that honors the transition as an evolution, not an emergency?
Oh wow. So the only thing worse than menopause… is being told you can’t even try to fix it? Thanks for the guilt trip, Dr. Clinical Trial. Meanwhile, I’m over here taking a $200/month compounded enclomiphene cocktail with ‘supportive herbs’ because my OB-GYN just handed me a pamphlet on ‘lifestyle changes’ and told me to ‘embrace the change’. Embrace it? I’m 58 and I just want to sleep through the night without feeling like I’m in a sauna made of regret.
my dr gave me fezolinetant and i cried because it worked. like, actually worked. no more 3am panic sweats. i didn’t think anything would. i thought i was stuck being a human radiator. this isn’t magic. it’s science. and it’s okay to be grateful for science.
Just wanted to say thank you for this. I’m a nurse and I’ve had so many women come in crying because they’ve been sold a dream that doesn’t exist. This post is going straight to my patient handout folder. You made the complex simple and the scary manageable. Seriously - thank you.
As a man who’s watched my wife go through this, I want to say - this isn’t just about hormones. It’s about identity. It’s about being seen. The drugs help, sure. But what helped her most was when I stopped saying ‘it’s just menopause’ and started asking ‘what do you need today?’ Maybe the real treatment isn’t in a pill - it’s in a listening ear, a cool towel, and someone who doesn’t rush you to ‘get over it’.